Sinoright Blog
Pvoidone-iodine
Povidone-iodine is a chemical complex of the polymer povidone (polyvinylpyrrolidone) and triiodide.
It is soluble in cold and mild-warm water, ethyl alcohol, isopropyl alcohol, polyethylene glycol, and glycerol. Its stability in solution is much greater than that of tincture of iodine or Lugol's solution.Free iodine, slowly liberated from the povidone-iodine (PVP-I) complex in solution, kills cells through iodination of lipids and oxidation of cytoplasmic and membrane compounds. This agent exhibits a broad range of microbiocidal activity against bacteria, fungi, protozoa, and viruses. Slow release of iodine from the PVP-I complex in solution minimizes iodine toxicity towards mammalian cells.


Povidone-iodine is a broad spectrum antiseptic for topical application in the treatment and prevention of wound infection. It may be used in first aid for minor cuts, burns, abrasions and blisters. Povidone-iodine exhibits longer lasting antiseptic effects than tincture of iodine, due to its slow absorption via soft tissue, making it the choice for longer surgeries. Chlorhexidine provides superior results with equivalent adverse events.
How to test Povidone-iodine purity:
A: Add 1 drop of the solution (1 sample, 10 parts water) to a mixture of 1 ml of starch indicator and 9 ml of water: the solution
will appear dark blue.
B: Spread 1 ml of the solution (1 sample, 10 parts of water) on a glass plate with an area of about 20 cm*20 cm and let it stand in low humidity air.
Air dry overnight at room temperature in low humidity air: brown, dry, non-smearing liquid film formation, in water
Gradual dissolution in water.
Loss on drying - Take 5.0 g of sample and dry at 105°C until the difference in weight after 2 consecutive dryings (1 hr apart) does not exceed 5.mg.
The weight loss on drying should not exceed 8.0%.
Burning residue - take 2g of sample, the result is negligible.
Iodine ions -
Determine total iodine - dissolve approximately 500 mg of povidone iodine (accurately weighed) in 100 ml of water in a 250 ml conical flask.
Add sodium bisulfite until the color of the iodine disappears. Add 25.0ml of 0.1N silver nitrate and 10ml of nitric acid and mix.
Reverse titrate the excess silver nitrate solution with 0.1N ammonium thiodicarbonate solution, using ammonium iron sulfate indicator as an indicator. Perform a
Perform a blank titration. Each 1 ml of 0.1N silver nitrate solution is equivalent to 12.69 ml of iodine. Calculate the percentage of total iodine on a dry basis.
Subtract the percentage of effective iodine (see Effective Iodine Content) to obtain the percentage of iodine ions. On a dry basis, the iodine ion content should not exceed 6.6%.
content should not exceed 6.6%.
Heavy metals - 0.002%.
Nitrogen content - on a dry basis, the N content should be between 9.5% and 11.5%.
Effective iodine content - accurately weigh 5g of povidone iodine, place it in a 400ml beaker and add 200ml of water.
Cover the beaker and stir it mechanically at room temperature to dissolve it as much as possible, but not longer than
1 hour. The titration is carried out immediately with 0.1N sodium thiosulfate and 3ml of starch indicator is added to determine the end point of the titration. Simultaneously
Do a blank test and any necessary calibration. Each 1ml of 0.1N sodium thiosulfate is equivalent to 12.69mg of iodine.
Note: Matters that should be noted during the testing process such as effective iodine content testing method
1.Effective iodine calculation formula:
Effective iodine (%) =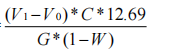
Effective iodine (%) =
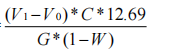
V1 = volume of standard solution of sodium thiosulfate (Na2S 2O 3-5H2O) consumed for titration of the sample (ml)
V0 = volume of standard solution of sodium thiosulfate (Na2S 2O 3-5H2O) consumed for titration blank (ml) C = concentration of standard solution of sodium thiosulfate (Na2S 2O 3-5H2O)
12.69 = Each ml of 0.1N sodium thiosulfate standard solution is equivalent to 12.69 mg of iodine
G = mass of the sample weighed (g)
W = weight loss on drying (%)
2. Example of calculation method.
Effective iodine (%) =
3. Preparation of standard solution: The factors affecting the PVP-I data are the selection of the standard solution, its configuration, the grade and the control of the various stages of the test.
The factors affecting the PVP-I assay data include the selection of standard solution, configuration, grade and control of each assay. For example, in this case: sodium thiosulfate standard solution needs to be stored for more than 2 weeks after configuration before calibration.
filtering is required before calibration.
4. the validity of the standard solution: the above-mentioned standard solution of sodium thiosulfate is valid for 2 months.
5. the storage of samples, sampling time, etc. will also have a certain impact on the test data. 6.
6. When taking samples, it should be noted that the sampling tube should be inserted into the bottom of the barrel. After taking the sample, mix the sample evenly before testing. Can not
Only take the surface sample inside the barrel, then the test result will be wrong.
When testing the effective iodine content of a sample, strictly follow the USP26 version of the effective iodine content test method.
In particular, the following should be noted.
a. The beaker should be kept covered while the sample is stirring in the beaker.
b. Water should be subtracted from the calculation of the effective iodine content.
c. The sample should be stirred mechanically at room temperature within 1 hour (not to exceed 1 hour) to dissolve the sample as much as possible.
otherwise the effective iodine content will be low.
Note: The stirring time should be as close to 1 hour as possible, otherwise the effective iodine content will not be fully dissolved.
8. Sample storage.
a. Keep the sample sealed at room temperature and avoid sunlight; seal the sample as soon as possible after sampling.
b. Do not mix with other samples.
c. Opened samples should not be stored for too long.

3. Preparation of standard solution: The factors affecting the PVP-I data are the selection of the standard solution, its configuration, the grade and the control of the various stages of the test.
The factors affecting the PVP-I assay data include the selection of standard solution, configuration, grade and control of each assay. For example, in this case: sodium thiosulfate standard solution needs to be stored for more than 2 weeks after configuration before calibration.
filtering is required before calibration.
4. the validity of the standard solution: the above-mentioned standard solution of sodium thiosulfate is valid for 2 months.
5. the storage of samples, sampling time, etc. will also have a certain impact on the test data. 6.
6. When taking samples, it should be noted that the sampling tube should be inserted into the bottom of the barrel. After taking the sample, mix the sample evenly before testing. Can not
Only take the surface sample inside the barrel, then the test result will be wrong.
When testing the effective iodine content of a sample, strictly follow the USP26 version of the effective iodine content test method.
In particular, the following should be noted.
a. The beaker should be kept covered while the sample is stirring in the beaker.
b. Water should be subtracted from the calculation of the effective iodine content.
c. The sample should be stirred mechanically at room temperature within 1 hour (not to exceed 1 hour) to dissolve the sample as much as possible.
otherwise the effective iodine content will be low.
Note: The stirring time should be as close to 1 hour as possible, otherwise the effective iodine content will not be fully dissolved.
8. Sample storage.
a. Keep the sample sealed at room temperature and avoid sunlight; seal the sample as soon as possible after sampling.
b. Do not mix with other samples.
c. Opened samples should not be stored for too long.
